What is Citric Acid | Uses | Safety | Side Effects | 16 FAQs
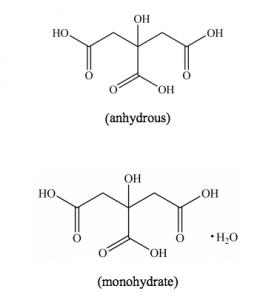
Citric acid anhydrous or monohydrate, the most widely used acidulant to give a sour taste in food and beverage, also acts as a preservative, PH buffer, antioxidant and chelating agent. The European food additive number for it is E330. China is the biggest manufacturer of citric acid in the world and exported around 1 million tons in 2019.
What is citric acid?
Two types in market
Natural sources
How is it made?
It is a weak organic acid naturally found in citrus fruits, providing the acidic taste and makes citrus fruits taste sour. Also, it plays an important part in the metabolism of most living things as an intermediate in the tricarboxylic acid cycle or Krebs cycle.
Two types in market
There are two forms of citric acid sold in the market, anhydrous and monohydrate. Monohydrate can turn to anhydrous in the condition of heated above 37 °C.

Natural sources
Citrus fruits have a high level of citric acid. Fruit containing citric acid includes:
Lemon
Lime
Pineapple
Oranges
Tomatoes
Strawberries
Peaches
Apples
Grapefruits
Blueberries
Bananas
Concentration
Lemon juice and lime juice are rich in citric acid. The concentration is around 41 mg/kg in ready-to-consume lemon juice and around 31 mg/kg in concentrates. The numbers are 39 mg/kg and 30 mg/kg in lime juice. In commercial lemon juice-based drinks, such as lemonade, the content ranges from 0.62 to 0.96 mg/kg. (1)
How is it made?
Citric acid is commercially produced by microbial fermentation of a glucose or sucrose carbohydrate substrate derived from corn.
Generally made from feeding substrate to black mold. Aspergillus niger is the major organism used in the microbial production of citric acid.
There are three manufacturing processes approved by the FDA as follows (2):
1.From plant sources, e.g. lemon or pineapple juice.
2.By mycological fermentation using Candida spp.
3.From Aspergillus niger fermentation liquor.
A study published in Toxicology Reports in 2018 said that approximately 99% of the world’s output of citric acid is using the fungus Aspergillus niger since 1919. So we can conclude that the third process method above is the most used one. (3)
Specification
| Other Names |
|
| CAS Number |
|
| Chemical formula |
|
| Molecular Weight |
|
| Melting Point | 156 °C |
| Boiling point | 310 °C |
Properties
PKa
PH
Solubility
Appearance
A white crystalline powder or granular with a strongly acidic taste.
Pka
PKa is used to describe the acid dissociation. Citric acid is a weak organic acid containing three carboxylic acid functional groups and as a result it has three PKa values, PKa1 = 3.14, PKa2 = 4.77 and PKa3 = 6.39.
PH
PH measures the concentration of H+ ions or H3O+ ions of the citric acid solution. The PH value depends on the citric acid concentration and its dissociation. It is indicated that the PH value of citric acid is 2.62 in the concentration of 10 mM (0.01mol/L) (4)
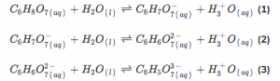
How to calculate the PH of citric acid?
Step 1: Write the dissociation equation.
We should do a systematic calculation involving all three equations as citric acid is a tricarboxylic acid.
Step 2: We could only calculate the first dissociation as it is a weak acid.
[H3O+] is the Hydronium concentration
[C6H7O7−] is the conjugate base concentration
[C6H8O7] is the citric acid concentration
The concentration of [C6H7O7−] and [H3O+] is the same. And we suppose the concentration is X. And the final concentration of [C6H8O7] is 0.01 – X.
Step 3: Then the equation can be written X*X/0.01-X = Ka1=10^-3.14.
Please be attention, the equation cannot be similar to X*X/0.01 = 10^-3.14 as X cannot be negligible compared with 0.01.
Step 4: Check for negligibility
0.01/10^-3.14=10^1.14, so it should be calculated in.
Step 4: calculate the equation and we can get the X=10^-2.62. So the PH value is 2.62.
Solubility
In water
Anhydrous: has a high solubility in water (592 g/L, 20 °C)
Monohydrate: soluble in water
In organic solvents
Anhydrous: soluble in ethanol and sparingly soluble in ether; Monohydrate: sparingly soluble in ether.
What are the health benefits?
Citric acid can help our body work against oxidation, also it can prevent kidney stones and is good for our skin.
Body antioxidation
Prevent kidney stones
Skin Benefits
Body antioxidation
Citric acid is an antioxidant that may protect human cells against the effects of free radicals.
Prevent kidney stones
Citric acid is beneficial for people with kidney stones by inhibiting the new stone formation and breaking down small stones at the start of forming. Its salts calcium citrate and potassium citrate also have stone prevention effects. (5)
Skin Benefits
Citric acid functions as an alpha-hydroxy acid (AHA) in skin care products. AHA consists of a carboxylic acid and a hydroxyl group on the adjacent carbon and is used widely in the cosmetics industry.
Generally, AHA can improve skin conditions, such as make fine lines and surface wrinkles smooth, improve skin texture and tone, unblock and clean pores. (6)
What are the common uses?
Citric acid can be used in a range of applications, including food and beverages, pharmaceuticals, cosmetics, water treatment, and household detergents due to its multifunctional characteristics.
Food
It is the common organic acid added to food together with malic acid and tartaric acid. Food grade citric acid can be used for an acidulant, preservative, antioxidant and chelating agent in food.
Acidulant
It is commonly used to add an acidic (sour) taste, enhance flavor and adjust PH to foods and soft drinks.
Preservative
Due to its acidic pH, it is used for food preservation as many bacteria cannot grow in an acidic environment.
Antioxidant
When present at low concentrations, it can prevent or slow down the oxidation process in foods.
Chelating agent
It can improve the quality and stability of foods with the ability to form chelate complexes with polyvalent metal ions.
Nutrition supplement
Its derivatives, calcium citrate and ferric citrate can be used as the supplement of calcium and iron to foods.
Cosmetics
Per the “European Commission database for information on cosmetic substances and ingredients”, it functions as a buffering, chelating and masking agent in cosmetic and personal care products. (7)
We can find some cosmetics with it:
Skin care products: face serum
Hair products: shampoo
Mouthwash
Pharmaceutical
Citric acid can chelate metal ions, buffer pH and impart flavor in the pharmaceutical industry. It can also be used as an external anticoagulant as it can prevent blood coagulation by lowering the concentration of calcium ions in blood through binding with calcium ions which will form a soluble complex but difficult to dissociate.
Industry uses
Water softener
Citric acid’s ability to chelate metals makes it used as a water conditioner in the treatment of industrial water and drinking water. Like EDTA, it is a chelating agent that can bind metal ions, commonly calcium and magnesium in water and remove them from pipes, household appliances, and etc.
Household cleaner
Citric acid can be used in household cleaning products, acting as a multifunctional cleaner for dishwasher and window, also for dish soaps, laundry detergents, rust removers and so on.
Is citric acid safe to eat?
Yes, its safety when used as a food additive has been approved by the U.S. Food and Drug Administration (FDA), European Food Safety Authority (EFSA), Joint FAO/WHO Expert Committee on Food Additives (JECFA), as well as other authorities.
FDA
Citric acid is generally considered safe (GRAS) and can be used in food with no limitations other than current good manufacturing practice (8). It can be used as an antimicrobial agent, antioxidant, flavoring agent, ph control agent, sequestrant in food. (9)
EFSA
Citric acid anhydrous and monohydrate (E330) are authorised as food additives in Commission Regulation (EU) No 231/2012 and categorized as “additives other than colours and sweeteners” (10)
Uses
The maximum level of E330 is “quantum satis”, which means there is no specific limit in its uses. The following foods may contain with it (11):
Mozzarella, whey cheese
Fats and oils
Frozen fruit and vegetables, canned or bottled fruit and vegetables
Jam, jellies and marmalades
Cocoa and chocolate products
Pasta, fresh minced meat, unprocessed fish
Table-top sweeteners in liquid/powder/tablets form
Infant formulae, processed cereal-based foods and baby foods
Fruit juices, beer and malt beverages
UK Food Standards Agency
Categorized in “Others” (12)
Food Standards Australia New Zealand
It is an approved ingredient with the code number 330 in Australia and New Zealand. (13)
JECFA
Functional class: Food Additives, flavouring agent, acid, antioxidant synergist, sequestrant. (14)
Acceptable daily intake: ADI “not limited” set since 1973 for it and its salts. (15)
What are the possible side effects?
It is common that sometimes consumers have concerns if the ingredient citric acid is bad for our health and what are the dangers. It is generally considered safe but some people may be allergic to it.
Allergy & Intolerance
Some people may be sensitive to citric acid and following allergy symptoms may occur (16):
Hives
Difficulty breathing
Swelling of your face, lips, tongue, or throat.
Other Possible Side Effects
A report in 2018 found that foods, beverages or vitamins containing citric acid may cause following side effects in some people:
Respiratory symptoms
Joint pain
Irritable bowel symptoms
Muscular pain and enervation
Tooth erosion
The intake of acid-containing food may lead to tooth erosion which is a chronic loss of dental hard tissues, enamel and dentine. A study published in 2015 found that the acidic beverage, e.g. apple juice and orange juice which containing citric acid are about five times more erosive than Coca-Cola light. (17)
Does it cause cancer?
No, in contrast, a study published in Cell Journal in 2016 found that citric acid prevents esophageal cancer 109 cell growth by inhibiting cell proliferation and inducing apoptosis (18). Also, Nature reported that citrate can suppress tumor growth. (19)
Is it safe for pregnant?
It is generally safe, better consult your doctor.
Frequently asked questions
Is it polar?
Is it ionic or covalent?
Is it an amino acid?
Is it a sugar?
Is it natural?
Is it organic?
Is it vegan?
Is it halal?
Is it kosher?
Is it gluten free?
What are the substitutes?
Citric acid Vs sodium citrate?
Does coffee have citric acid?
Is citric acid the same with vitamin C?
What is encapsulated citric acid?
Comparison among citric acid, malic acid, tartaric acid, acetic acid, and phosphoric acid
What is encapsulated citric acid?
Is it polar?
Yes, it is polar and soluble in polar substance, e.g. water.
Is it ionic or covalent?
It is a covalent compound and no ionic bonds.
Is it an amino acid?
No, is citric acid an amino acid nor it contains amino acids.
Is it a sugar?
No, but it can be fermented from sugar substrate by the black mold Aspergillus niger.
Is it natural?
Yes, It can be considered natural as it occurs naturally in citrus fruits, like lemons and limes and commercially produced by the fermentation process as mentioned above instead of utilizing the extraction method.
Is it organic?
It can be organic if from the organic raw materials source.
Is it vegan?
Yes, as mentioned above, it is vegan as the raw material glucose and/or sucrose carbohydrate used and manufacturing process without the use of animal matter or products derived from animal origin. As a food ingredient, it is considered vegan and can be added in the food for vegetarians.
Is it halal?
Yes, it is generally recognised as halal as it is permitted under the Islamic Law and fulfill the conditions of Halal. And we can find some manufacturers certificated with MUI halal.
Is it kosher?
Yes, it is kosher pareve. E330 has met all the “kashruth” requirements and can be certified as kosher or passsover.
Is it gluten free?
Yes, it is typically gluten-free and people with celiacs can eat it. It is an ingredient commonly found in both gluten-free and gluten-containing food labels. It is produced from corn complying with the FDA’s definition of gluten free, that it does not contain wheat, rye, barley, or crossbreeds of these grains.
What are the substitutes?
Other organic acids like malic acid, tartaric acid and fumaric acid can be the alternatives in some food applications.
Citric acid Vs sodium citrate?
Sodium citrate is the sodium salt of citric acid. It is produced by citric acid reaction with sodium hydroxide or baking soda.
Sodium citrate has three forms, monosodium citrate, disodium citrate and trisodium citrate, commonly it refers to last one.
Following is the comparison and difference:
Acidity: Citric acid is an organic weak acid while sodium citrate is a weak alkaline.
Taste: Citric acid is mainly used to enhance the flavor with a sour taste while the sour taste of sodium citrate is not as obvious as citric acid. The sour taste occur when it is hydrolysis to citric acid.
Uses: There is no much difference in both applications. Both can be used as a preservative, PH buffer and chelating agent in food.
Does coffee have citric acid?
Yes, coffee has it inside. (20)
Is citric acid the same with vitamin C?
No, they’re both organic acids. Vitamin C is also called ascorbic acid. Although citric acid and ascorbic acid both occur naturally in citrus fruits, they’re two totally different ingredients.
Uses: Ascorbic acid is a vitamin mainly used as a nutritional supplement, antioxidant and color protection agent in food while citric acid is an acidulant used as PH control, preservative, antioxidant and chelating agent.
Manufacturing: both manufactured from fermentation but with different processes and microbial.
What is encapsulated citric acid?
It has a specific application. It is citric acid with a coating outside which prevents the citric acid from releasing in advance.
Comparison among citric acid, malic acid, tartaric acid, acetic acid, and phosphoric acid?
They are all acidulants used in food and drink. Following are the brief comparison among them:
Sources: they’re all the organic acid can be found in most fruits except phosphoric acid which is an inorganic acid.
Types: Both malic acid and tartaric acid has L and DL types used as food additives.
Manufacturing process:
Citric acid, L-malic acid, comes from glucose fermentation.
L-tartaric acid can be obtained in the way of a winemaking byproduct, enzyme process, or fermentation method.
DL-malic acid, acetic acid and phosphoric acid are derived from chemical synthesis.
DL-tartaric acid is produced from the enzyme.
Form: the first three acidulants are available both in powder and granular form, while the rest two are liquid.
Conclusion
Now you may have a good knowledge of the food additive – citric acid (E330), from:
Two types: monohydrate and anhydrous
Production
Uses as an acidity regulator, preservative, antioxidant and chelating agent in food
Health benefits
Approved safety
Possible side effects
FAQs such as is it vegan, gluten free, synthetic or natural
Comparison with other acidity regulators: malic acid, tartaric acid, acetic acid, and phosphoric acid
What kinds of food packaging have you found this ingredient in? Let me know in the comments.
Post time: Jun-09-2021

